Adjusting and Measuring pH Levels in Your Aquarium: A Comprehensive Guide
Quick Links – Key Topics
Brief Description
This article provides a comprehensive guide on measuring and adjusting pH levels in your aquarium. For more related information, explore our other articles: Normal pH Levels in Tropical Fish Tanks, Aquarium Water Chemistry FAQ, Benefits of Using Distilled Water in Aquariums, and Water Hardness Explained. If you have further questions, please use the form at the bottom of this page to ask, and we'll be happy to assist!
Introduction
pH, which measures the acidity or basicity of a solution, is a crucial factor that should be regularly checked in any fish tank, ideally once a week. Monitoring pH helps prevent unexpected losses of fish and plants. Before we explore how to adjust pH levels in an aquarium, let's review some basic guidelines:
- There isn’t a universally “ideal” pH value, but a pH of 7 is generally suitable for most fish species, regardless of their origin. Even many African cichlids can tolerate a pH of 7, though it’s best to replicate their natural environment as closely as possible. In fish tanks, pH typically ranges from 6 to 8, though it can technically range from 0 to 14.
- Performing basic pH tests is essential before buying new fish or plants. Ask store staff about the pH in the tanks where your new fish or plants are kept before making a purchase. Ensure that the pH in your home tank is similar to the pH in the store tanks. A difference of up to 0.5 is usually safe.
- Carbonate hardness influences pH levels. If carbonate hardness drops too low (0-3), pH will become unstable and unpredictable until carbonate hardness is raised to 4 or higher.
- A pH of 7 indicates neutral water. Simply put, if pH levels drop below 7, acidity in the water increases, while a pH above 7 means the water becomes more alkaline. It’s important to avoid significant fluctuations, as they can be harmful to fish.
How to Increase pH in a Fish Tank
The simplest way to raise the pH in your fish tank is by adding a few limestone rocks. These rocks gradually release calcium, which not only boosts pH but also hardens the water. To check if a rock contains enough calcium, pour vinegar on its surface - if it bubbles, it's suitable for raising pH and increasing water hardness. Ensure that the rocks are smooth and flat to avoid injuring your fish.
Another straightforward method is to place crushed calcium sources in your filter’s media chamber. Canister filters (whether external or HOB) are perfect for this, as they provide ample space for additional materials. Many aquarium stores sell crushed coral, which can be added to the filtration chamber. It doesn't require much room, and you can mix it with other filter media like ceramic rings or activated carbon.
A third approach, related to the previous ones, involves adding limestone rocks and coral to a separate tank that doesn’t house any fish. In this tank, the pH can easily rise to 9. This tank doesn’t need filtration, but covering it with a lid helps keep out dust and debris. Use water from this temporary tank when performing water changes in your main aquarium. Be sure to monitor the pH in the temporary tank before using the water. One benefit of this method is that chlorine will dissipate from the temporary tank over time. Another advantage is that you can control the pH in your primary tank by gradually adding small amounts of this “high pH water” and testing the levels after each addition.
For a quick pH increase, you can add sodium bicarbonate (baking soda) to your aquarium. This not only raises the pH but also increases carbonate hardness, addressing two issues at once. However, you must add sodium bicarbonate carefully, as it needs to dissolve fully. The recommended amount is 1 teaspoon per 50 liters (13 US gallons, 11 Imperial gallons) of water. Here’s how to do it:
- First, measure the pH and carbonate hardness in your aquarium. If the levels are unsatisfactory, proceed to the next step.
- Mix chlorine-free water with sodium bicarbonate in a glass (e.g., 1 teaspoon per 50 liters, so if your tank holds 400 liters, use 8 teaspoons).
- Slowly pour the mixture into your aquarium.
- After 10 minutes, test the pH and carbonate hardness again. If the results are still not satisfactory, repeat the process.
Another option is to use commercial products designed to raise pH, but be cautious. These products usually don't increase carbonate hardness (KH), which means the pH might drop again within a day or two.
How to Reduce pH in a Fish Tank
One effective way to lower the pH in your fish tank is by using peat in the filtration chambers. However, it's crucial to use chemical-free peat, so it's best to purchase peat from aquatic stores rather than using garden peat, even though many garden products are now 100% safe for aquariums. Place the peat in the filtration chamber, but keep in mind that it won't lower the pH as quickly as sodium bicarbonate can raise it. Another natural method for reducing pH is adding driftwood to the tank. Driftwood will gradually lower the pH over time, but the speed of this process can vary depending on factors such as tank size, the number of fish and plants, substrate, and other objects that affect pH and carbonate hardness.
If the pH of your tap water is significantly lower than that of your aquarium (for example, if the tap water has a pH of 7 and the aquarium water has a pH above 7.8), using aged, chlorine-free tap water can be an effective way to reduce pH. Chlorine typically evaporates within 24 hours after filling a bucket with tap water, making this a simple method to consider.
If the pH in your tank remains too high, you might need to use a reverse osmosis unit to remove some of the minerals. This is one of the safest ways to make your water more acidic, although reverse osmosis units can be expensive.
Commercial products designed to lower pH should be considered a last resort.
Exceptions
As with most rules, there are exceptions when it comes to controlling pH in aquariums. For instance, fish from South America tend to thrive in softer water with higher acidity and can do well in water with a pH level of around six. Conversely, East African fish prefer hard, highly alkaline water and do best with a pH of eight or more. The key is to find a variety of fish that can thrive at the target pH level of seven and build your aquarium around that.
Examples of Fish Species with Different pH Requirements:
Discus, Angelfish, Rams, and Apistogrammas all need soft, acidic water, with pH levels below 7.0. If you cannot maintain this pH level, it might be worth considering other species. Tank-bred specimens may tolerate slightly higher pH levels, but for wild specimens, higher pH levels can be harmful to their health.
On the other hand, Guppies and Swordtails prefer alkaline water, requiring a pH above 7.0. In this case, you may need to buffer your water to maintain a sufficiently high pH. It's important to remember that when a tank is first set up, the pH will be at its highest. After a few weeks, the pH may drop slightly, so regular water testing is crucial to monitor this.
It's also important to note that over time, the acidity of the water may gradually increase. This slow process typically doesn't cause significant issues, as vigilant aquarists can manage it through proportional water changes as part of their regular maintenance routine.
Monitoring Other Water Parameters: In addition to pH, other aspects of water quality, such as hardness or the presence of chemicals, need to be regularly checked. Unfortunately, no single test kit covers all these important parameters, so it’s essential to have separate kits for each type of test, including those for pH adjustments.
Carbonate Hardness
Water naturally has the ability to resist changes in its pH level, a feature known as a buffering system. Aquarists interested in understanding the capacity of their aquarium's buffering system can do so by measuring the carbonate hardness of the water, which is expressed in dKH (distinct from dGH, which measures overall hardness).
Once the water hardness levels are determined, you'll find that they tend to remain fairly consistent. A carbonate hardness (dKH) level between four and six is usually enough to keep the buffering system of a typical aquarium stable. However, as mentioned earlier, a carbonate hardness of three or lower is insufficient, leading to an unstable buffering system that may cause pH levels to drop unexpectedly.
Short Summary
There isn’t a single “ideal” pH for aquariums; rather, each species of fish has its own ideal pH level. Different fish come from diverse habitats around the world, each with its own unique water conditions. This means that pH requirements can vary significantly among tropical fish species.
A Guide to Testing pH in Your Aquarium
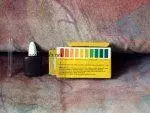
Start by purchasing a pH test kit, similar to the one shown below (this also applies to testing ammonia, nitrates, and nitrites).
Next, follow the instructions provided by the manufacturer carefully.
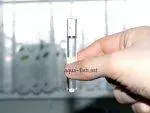
In this example, the pH of the water was quite low (around 5.8). This suggests that a water change might be necessary. We then performed another test (note that the result showed a green color at the top of the test tube instead of black).
After adjustment, the pH level was around 7.1-7.2, which is ideal for most aquarium fish species. It’s also important to test water hardness. The following images demonstrate how straightforward this process can be.
Additional Questions and Answers
As of March 22, 2011, we have included the following questions here due to the integration of aqua-fish.net/answers with our articles. This consolidation aims to provide all related information in one place. Feel free to ask your own unique questions that haven't been answered on this page yet. Use the form at the bottom to submit your questions.
-
What pH level should be in an aquarium?
Answer: A pH level of 7 is suitable for most aquariums. However, there are exceptions. For instance, African Cichlids typically require a higher pH of around 8, while some South American biotopes need a lower pH of around 6.
-
What causes pH to drop?
Answer: A drop in pH is often due to low dGH (general hardness). When dGH is below 4, pH levels become less stable. Additionally, a lack of minerals in the water can also contribute to a lower pH.
-
What happens to aquarium fish when the pH is too high or too low?
Answer: If the pH is too low for the fish species, you might observe symptoms like gill burning and darkening of the scales. This stress can lead to health issues. Conversely, a high pH can stress fish species accustomed to acidic conditions, weakening their immune systems and causing them to suffer.